Introduction:
Piramal Pharma jobs for QC QA IPQA Process Validation RM FG Stability BPharm MPharm MSc are opening doors for skilled pharmaceutical professionals looking for career growth in a reputed global company. This walk-in interview drive offers excellent roles for both experienced candidates and freshers in quality and validation functions. If you are aiming to work in a well-established pharma organization with international standards, this opportunity is highly valuable.
Company overview:
Piramal Pharma jobs for QC QA IPQA Process Validation RM FG Stability BPharm MPharm MSc come from Piramal Pharma Limited, a well-known pharmaceutical company with strong global footprints and advanced manufacturing operations. The company is recognized for its commitment to quality, innovation, and compliance with international regulatory standards. Its Pithampur facility in Madhya Pradesh is equipped with modern infrastructure and focuses on delivering high-quality pharmaceutical products across global markets. Piramal Pharma provides a professional work culture, strong learning environment, and long-term career development for its employees.
Job details:
Piramal Pharma jobs for QC QA IPQA Process Validation RM FG Stability BPharm MPharm MSc offer multiple openings across quality control, quality assurance, and validation departments.
Job Title:
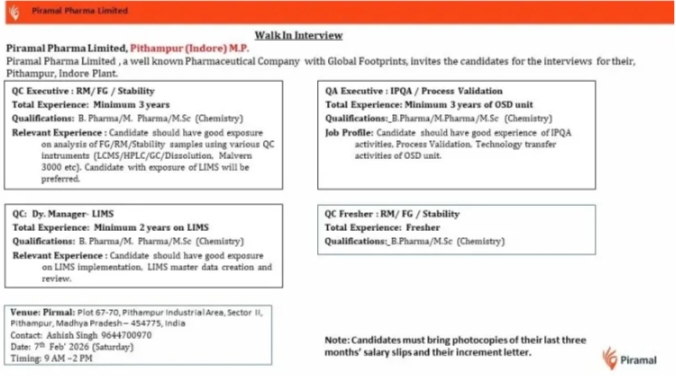
QC Executive
QA Executive IPQA Process Validation
QC Dy Manager LIMS
QC Fresher RM FG Stability
Department:
Quality Control
Quality Assurance
IPQA
Process Validation
LIMS
RM FG Stability
Salary:
N/A
Requirements:
Skills:
Strong knowledge of QC and QA processes
Experience in RM FG Stability sample analysis
Understanding of IPQA and Process Validation activities
Knowledge of technology transfer in OSD units
Hands-on exposure to LIMS software
Data management and documentation skills
Understanding of GMP guidelines
Good communication and teamwork skills
Qualification:
BPharm
MPharm
MSc Chemistry
Experience:
Minimum 3 years for QC Executive RM FG Stability
Minimum 3 years in OSD for QA Executive IPQA Process Validation
Minimum 2 years for QC Dy Manager LIMS
Freshers for QC RM FG Stability
Vacancies:
Not yet disclosed
Interview Details:
- 📁 Department: Quality Control
📄 Qualification: BPharm, MPharm, MSc Chemistry
📄 Experience: Minimum 3 years RM FG Stability - 📁 Department: Quality Assurance IPQA Process Validation
📄 Qualification: BPharm, MPharm, MSc Chemistry
📄 Experience: Minimum 3 years in OSD - 📁 Department: Quality Control LIMS
📄 Qualification: BPharm, MPharm, MSc Chemistry
📄 Experience: Minimum 2 years - 📁 Department: Quality Control RM FG Stability
📄 Qualification: BPharm, MPharm, MSc Chemistry
📄 Experience: Freshers
Type:
Walk-In Interview
Date and Time:
07-02-2026 Saturday
09:00 AM – 02:00 PM
Location/Platform:
Piramal Pharma Limited
Plot 57-70, Pithampur Industrial Area, Sector II
Pithampur, Madhya Pradesh – 454775
India
Documents Required:
Updated CV/Resume
Academic Certificates
Experience Certificates
Last Three Months Salary Slips
Increment Letter
ID Proof
Passport Size Photos
How to Apply:
Piramal Pharma jobs for QC QA IPQA Process Validation RM FG Stability BPharm MPharm MSc can be applied by directly attending the walk-in interview at the Pithampur venue on the scheduled date. Candidates should carry all required documents and reach the venue early. Dress professionally and be prepared to discuss technical knowledge related to quality systems, validation, and pharmaceutical manufacturing practices. This is an excellent opportunity to build a strong career with a globally recognized pharmaceutical organization offering growth, learning, and stability.
 Follow us on LinkedIn
Follow us on LinkedIn
