Macleods Pharmaceuticals is conducting a large walk-in hiring drive at Chhatrapati Sambhajinagar for multiple regulated manufacturing units, offering career opportunities across Quality Control, Production, Engineering Services, Stores, and API operations. This walk-in interview is open to experienced pharma professionals with hands-on exposure to formulation, injectables, API manufacturing, quality systems, and plant maintenance, making it a strong opportunity for candidates seeking growth in a well-established pharmaceutical organization.
About the Company
Macleods Pharmaceuticals Limited is a globally recognized pharmaceutical company with a strong presence in formulations, injectables, and active pharmaceutical ingredients. The company operates multiple GMP-compliant manufacturing facilities across India, supplying regulated markets worldwide. Macleods is known for its robust quality systems, strong regulatory compliance culture, and continuous focus on innovation and operational excellence. With manufacturing capabilities spanning OSD, dry powder injections, APIs, and quality laboratories, the organization emphasizes training, data integrity, and sustainable growth within a regulated pharma environment.
Job Details
• Company Name: Macleods Pharmaceuticals Limited
• Job Type: Walk-in Interview
• Experience Required: 2 to 8 years (department-wise)
• Qualification: BPharm/MPharm/BSc/MSc/ITI/Diploma/BE/BTech/BCom/MCom
• Location: Chhatrapati Sambhajinagar, Daman, Sarigam Unit V, Dahej, Baddi
• Work Profile: Production/Quality Control/Engineering Services/Stores/API Manufacturing
Job Description
Quality Control OSD
• Experience: 2 to 6 years
• Qualification: BPharm/MPharm/MSc Organic Chemistry
• Work Areas/Sections: Analytical validation, finished goods testing, raw material analysis, stability studies, GLP
✔ Exposure to regulated QC laboratory operations
✔ Handling analytical documentation and compliance activities
Engineering Services
• Experience: 2 to 8 years or 2 to 7 years
• Qualification: Diploma/BE/BTech Instrumentation/Electrical/Mechanical or ITI/Diploma Electrical/Mechanical
• Work Areas/Sections: Formulation plant maintenance, HVAC, instrumentation, electrical systems, water systems
✔ Preventive and breakdown maintenance activities
✔ Support to plant utilities and compliance systems
Production Dry Powder Injection
• Experience: 2 to 6 years
• Qualification: BPharm/MPharm or ITI/Diploma/BSc/Any Graduate
• Work Areas/Sections: Vial filling and sealing machines, crab system, autoclave, tunnel, vial washing, IPC, media fill
✔ Operation of injectable manufacturing equipment
✔ Adherence to aseptic practices and GMP
Stores API and Formulation
• Experience: 2 to 8 years
• Qualification: BSc/BCom/MSc/MCom/BPharm
• Work Areas/Sections: Receipt and dispensing, inward and outward material handling
✔ Inventory control and documentation
✔ Coordination with production and QA teams
Production Formulation OSD
• Experience: 2 to 8 years
• Qualification: BPharm/MPharm or ITI/Diploma
• Work Areas/Sections: Compression, packing, bulk packing, blister packing
✔ Operation of formulation equipment
✔ Compliance with batch documentation and GMP
Production API
• Experience: 2 to 8 years
• Qualification: BSc/MSc Chemistry/BE/BTech Chemical/Diploma Chemical
• Work Areas/Sections: API and intermediates manufacturing
✔ Handling API production processes
✔ Documentation and regulatory compliance
Quality Control API
• Experience: 2 to 6 years
• Qualification: MSc/BPharm/MPharm
• Work Areas/Sections: Analytical validation, finished goods, raw materials, stability testing, GLP
✔ API QC laboratory exposure
✔ Regulatory documentation handling
Quality Control MDI
• Experience: 2 to 6 years
• Qualification: MSc Organic Chemistry
• Work Areas/Sections: Finished products testing, raw material analysis, stability, packing material testing, AMT, AMV
✔ Quality testing for MDI products
✔ Compliance with quality standards
Skills/Qualifications
• Understanding of OSD, injectable, and API manufacturing operations
• Knowledge of GMP, GLP, safety practices, and documentation
• Awareness of equipment handling, aseptic techniques, and utilities
• Strong coordination and communication skills
• Regulatory and compliance awareness in pharma operations
Key Responsibilities
• Operating and monitoring production and utility equipment
• Performing quality control testing and documenting results
• Following GMP, safety, and cleanroom discipline
• Handling formulation, injectable, and API operations
• Supporting audits, validations, and shop floor activities
• Coordinating with QA, QC, Production, and Engineering teams
How to Apply
Interested candidates should attend the walk-in interview directly at the venue with required documents. Candidates must carry an updated resume, educational certificates, experience letters, salary structure with CTC breakup, and a valid Aadhaar card. Prior online registration through the QR code is required for participation.
Walk-in Interview Details
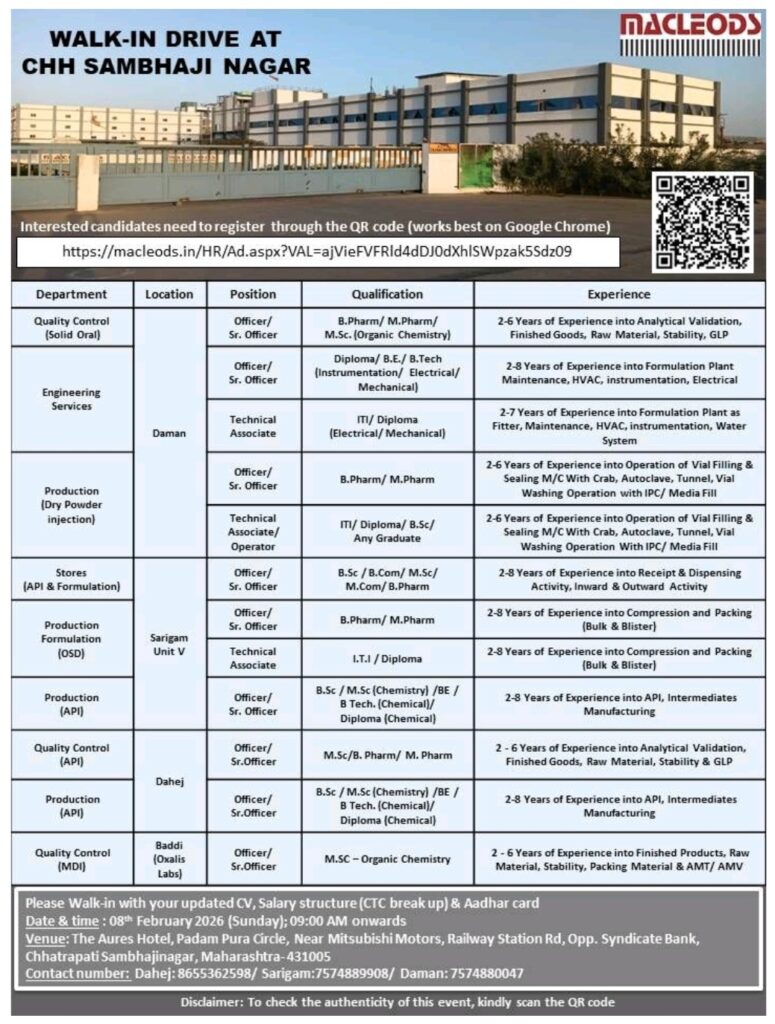
• Date: 08 February 2026 (Sunday)
• Time: 09:00 AM onwards
• Venue: The Aures Hotel, Padam Pura Circle, Near Mitsubishi Motors, Railway Station Road, Opp. Syndicate Bank, Chhatrapati Sambhajinagar, Maharashtra 431005
• Contact: Dahej – 8655362598, Sarigam – 7574889908, Daman – 7574880047
Why You Should Join
• Opportunity to work with a reputed global pharmaceutical company
• Exposure to regulated manufacturing and quality systems
• Strong GMP-driven and compliance-focused culture
• Career development across multiple pharma units
• Stable work environment with collaborative teams
FAQs
1. Which departments are hiring in this Macleods walk-in drive?
Quality Control, Production, Engineering Services, Stores, and API departments are hiring.
2. What is the experience requirement for these roles?
Candidates with 2 to 8 years of relevant pharma experience are eligible depending on the department.
3. What qualifications are accepted for this walk-in interview?
BPharm, MPharm, BSc, MSc, ITI, Diploma, BE, BTech, BCom, and MCom qualifications are accepted as per role.
4. Are freshers eligible for this walk-in interview?
No, this walk-in drive is for experienced candidates only.
5. Is API experience mandatory for API roles?
Yes, prior experience in API or intermediates manufacturing is required for API positions.
6. What type of production roles are available?
OSD formulation, dry powder injection, and API production roles are available.
7. What QC experience is required?
Experience in analytical validation, raw material testing, finished goods testing, and stability studies is required.
8. Where will the walk-in interview be conducted?
The walk-in will be conducted at The Aures Hotel, Chhatrapati Sambhajinagar.
9. What documents should candidates bring?
Updated resume, educational certificates, experience letters, CTC breakup, and Aadhaar card.
10. Is registration required before attending the walk-in?
Yes, candidates are required to register through the provided QR code before attending.
 Follow us on LinkedIn
Follow us on LinkedIn
