Piramal Pharma Limited pharma jobs walk-in interview at Pithampur Indore is a strong opportunity for experienced QA and QC professionals looking to grow in a reputed pharmaceutical organization. This hiring drive is focused on skilled candidates with solid exposure in OSD, stability, LIMS, process validation, and laboratory QA functions. If you are planning to switch or upgrade your pharma career in Madhya Pradesh, this walk-in interview can be the right move.
Company overview:
pharma jobs at Piramal Pharma Limited offer excellent career growth in a globally recognized pharmaceutical company with strong international footprints. Piramal Pharma Limited is known for its quality-driven manufacturing, regulatory compliance, and advanced analytical capabilities. The company operates modern manufacturing facilities and supports global markets with high standards of GMP and quality systems. Its Pithampur Indore plant is an important manufacturing location where experienced professionals contribute to QA, QC, and validation activities. The company focuses on innovation, compliance, documentation excellence, and continuous improvement in pharmaceutical operations.
Job details:
Job Title:
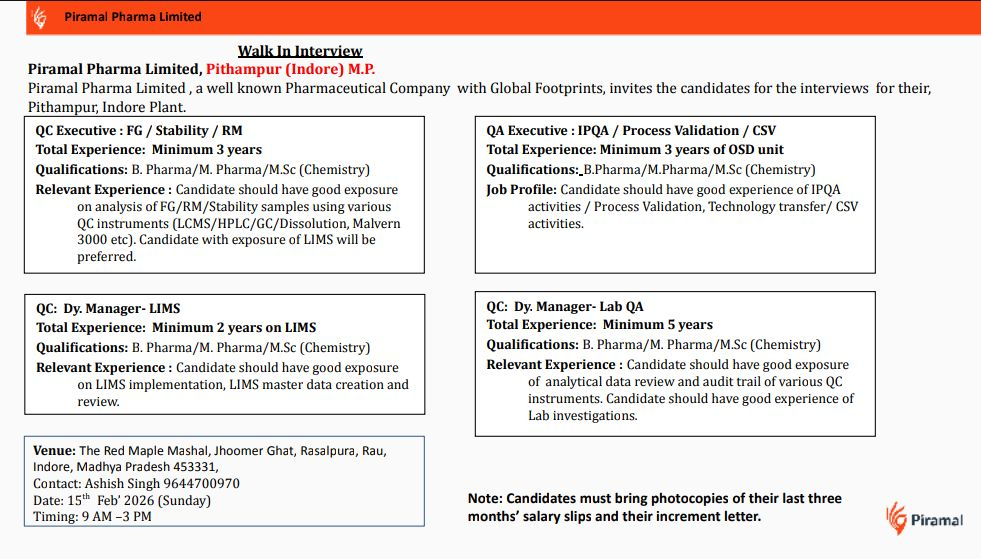
QC Executive FG Stability RM
QA Executive IPQA Process Validation CSV
QC Dy Manager LIMS
QC Dy Manager Lab QA
Department:
Quality Control, Quality Assurance
Salary:
N/A
Requirements:
Skills:
Experience in analysis of FG RM Stability samples
Hands on exposure to LCMS HPLC GC Dissolution Malvern 3000
Knowledge of LIMS implementation and master data creation
Experience in IPQA activities
Exposure to Process Validation and Technology Transfer
CSV activities knowledge
Analytical data review and audit trail review
Handling various QC instruments
Experience in lab investigations
Strong documentation practices
Understanding of GMP and regulatory compliance
Qualification:
BPharm, MPharm, MSc Chemistry
Experience:
Minimum 3 years for QC Executive FG Stability RM
Minimum 3 years of OSD unit experience for QA Executive IPQA Process Validation CSV
Minimum 2 years on LIMS for QC Dy Manager LIMS
Minimum 5 years for QC Dy Manager Lab QA
Vacancies:
Not yet disclosed
Interview Details:
- 📁 Department: Quality Control
📄 Qualification: BPharm MPharm MSc Chemistry
📄 Experience: Minimum 3 years FG Stability RM experience - 📁 Department: Quality Assurance
📄 Qualification: BPharm MPharm MSc Chemistry
📄 Experience: Minimum 3 years OSD IPQA Process Validation CSV experience - 📁 Department: Quality Control LIMS
📄 Qualification: BPharm MPharm MSc Chemistry
📄 Experience: Minimum 2 years LIMS implementation exposure - 📁 Department: Quality Control Lab QA
📄 Qualification: BPharm MPharm MSc Chemistry
📄 Experience: Minimum 5 years analytical data review and lab investigations experience
Type:
Walk-In Interview
Date and Time:
15th February 2026 Sunday
9 AM to 3 PM
Location/Platform:
The Red Maple Mashal, Jhoomer Ghat, Rasalpura, Rau, Indore, Madhya Pradesh 453331
Documents Required:
Updated CV Resume
Photocopies of last three months salary slips
Increment letter
Educational certificates
Experience certificates
ID proof
Passport size photographs
How to Apply:
Interested candidates can directly attend the walk-in interview at the mentioned venue on 15th February 2026. For any clarification, you may contact Ashish Singh at 9644700970. Candidates are advised to carry all required documents and arrive on time for smooth processing.
Piramal Pharma Limited pharma jobs in Indore provide a strong platform for QA and QC professionals to work in structured quality systems, regulatory-focused operations, and advanced analytical environments. If you meet the experience criteria, this walk-in drive can help you step into a stable and growth-oriented pharmaceutical career.
remember this FAQs
What is the minimum experience required for QC Executive FG Stability RM?
Minimum 3 years of relevant experience in FG RM Stability analysis using QC instruments like LCMS HPLC GC Dissolution and Malvern 3000 is required.
Is OSD experience mandatory for QA Executive IPQA Process Validation CSV role?
Yes, candidates must have minimum 3 years of experience in OSD unit along with IPQA, Process Validation, Technology Transfer and CSV exposure.
What experience is required for QC Dy Manager LIMS?
Minimum 2 years of hands on experience in LIMS implementation, master data creation and review is required.
What is the experience requirement for QC Dy Manager Lab QA?
Minimum 5 years of experience in analytical data review, audit trail review and lab investigations is required.
What qualifications are accepted for these roles?
BPharm, MPharm, MSc Chemistry candidates are eligible to attend the walk-in interview.
Are freshers eligible for this walk-in interview?
No, all roles require prior relevant experience as mentioned in the job details.
What documents should candidates carry for the interview?
Candidates must carry updated resume, photocopies of last three months salary slips, increment letter, educational certificates, experience certificates and ID proof.
What is the interview date and timing?
The walk-in interview will be conducted on 15th February 2026 Sunday from 9 AM to 3 PM at Indore.
 Follow us on LinkedIn
Follow us on LinkedIn
