NATCO Pharma has announced a walk-in interview for experienced and selected fresher candidates to strengthen its Parenteral division. This hiring drive focuses on core manufacturing, quality, engineering, warehouse, and microbiology functions, offering a strong opportunity for professionals who want to build or advance their careers in injectable and sterile pharmaceutical operations within a regulated environment.
About the Company
NATCO Pharma is a well-established Indian pharmaceutical company known for its expertise in complex generics, specialty pharmaceuticals, and injectables. The company operates advanced manufacturing facilities with a strong focus on parenteral and sterile dosage forms, supported by robust quality systems and compliance-driven operations. NATCO follows global regulatory standards such as cGMP and works within regulated markets, maintaining a culture of quality, continuous improvement, and technical excellence across its operations.
Job Details
• Company Name: NATCO Pharma Limited
• Job Type: Walk-in Interview
• Experience Required: Freshers to 12 Years (role-specific)
• Qualification: Diploma/BSc/BPharm/MSc/BTech/BCom/MBA
• Location: Hyderabad (Interview Location) | Work Location: Nagarjuna Sagar, Telangana
• Work Profile: Production/Engineering/Warehouse/Microbiology/Quality Assurance
Job Description
Production
• Experience: 2–12 Years (Freshers eligible for specific roles)
• Qualification: Diploma/BSc/BPharm/MSc
• Work Areas/Sections: Sterile injectable manufacturing, aseptic filling operations, production packaging, production QMS, SOP handling, manpower coordination, training activities
✔ Exposure to cGMP-compliant injectable manufacturing lines
✔ Roles include supervision, packaging operations, QMS support, and training-related activities
Engineering
• Experience: 2–6 Years
• Qualification: Diploma/BSc/BTech
• Work Areas/Sections: Lyophilizers, water systems, HVAC operations, instrumentation activities
✔ Hands-on maintenance and operational support for sterile facility equipment
✔ Involvement in critical utilities supporting parenteral manufacturing
Warehouse
• Experience: 4–5 Years
• Qualification: BCom/BSc/BPharm/MBA
• Work Areas/Sections: Raw material dispensing, material handling, warehouse QMS activities
✔ Practical exposure to dispensing processes in regulated environments
✔ Documentation and compliance-focused warehouse operations
Microbiology
• Experience: 2–6 Years
• Qualification: MSc Microbiology/Biotechnology
• Work Areas/Sections: Media preparation, disinfectant preparation, MLT, BET, sterility testing, method validation, water and bioburden testing
✔ Support to sterile manufacturing through microbiological monitoring
✔ Strong focus on documentation and compliance activities
Quality Assurance
• Experience: 3–12 Years
• Qualification: BPharm/MSc
• Work Areas/Sections: IPQA activities, QMS handling, validation activities
✔ Involvement in shop floor QA, validation execution, and quality systems
✔ Ensuring compliance with cGMP and internal quality standards
Skills/Qualifications
• Understanding of injectable and parenteral manufacturing operations
• Knowledge of cGMP, SOPs, documentation, and regulatory compliance
• Awareness of sterile area practices and cleanroom discipline
• Equipment handling knowledge relevant to production and engineering roles
• Strong communication, coordination, and teamwork skills
• Quality and compliance-oriented mindset
Key Responsibilities
• Operating and supporting sterile injectable manufacturing and filling operations
• Performing and reviewing in-process checks and maintaining accurate documentation
• Following cGMP guidelines, safety practices, and cleanroom discipline
• Handling validation, QMS, and microbiology-related activities as applicable
• Supporting audits, inspections, and continuous improvement initiatives
• Coordinating with Production, QA, Engineering, and Warehouse teams
How to Apply
Interested candidates should attend the walk-in interview in person. Carry an updated resume, educational certificates, experience letters, salary slips, and a valid ID proof. Candidates must be willing to work at the Nagarjuna Sagar location and in rotational shifts.
Walk-in Interview Details
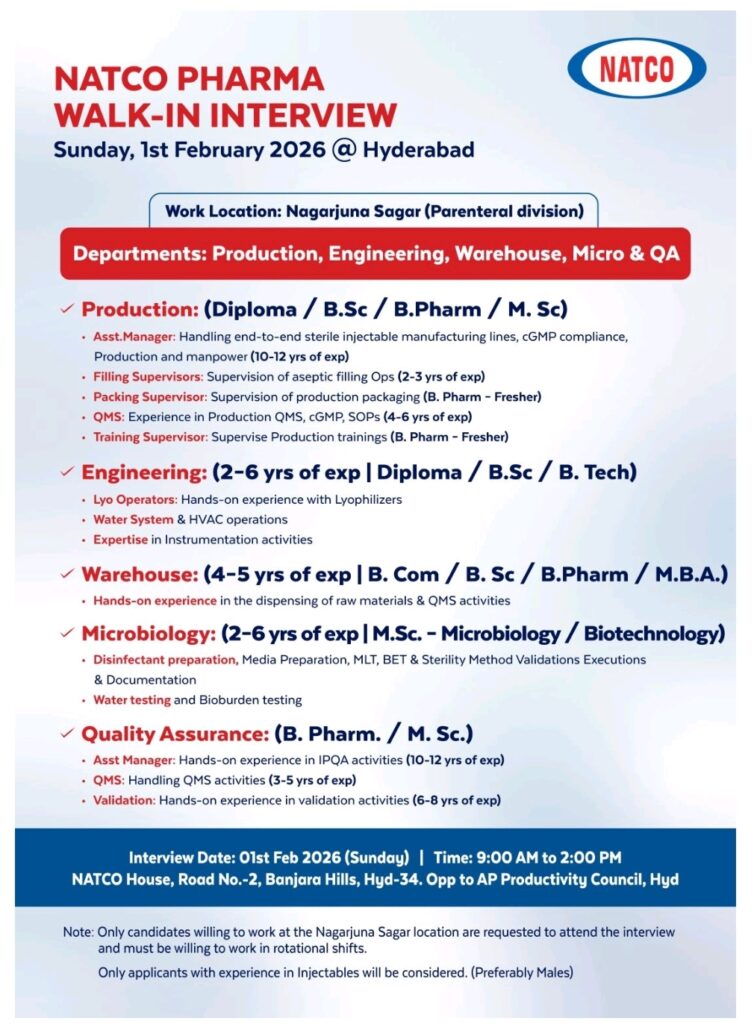
• Date: 01 February 2026 (Sunday)
• Time: 09:00 AM to 02:00 PM
• Venue: NATCO House, Road No. 2, Banjara Hills, Hyderabad – 34, Opposite AP Productivity Council
Why You Should Join
• Opportunity to work with a reputed pharmaceutical organization
• Exposure to sterile and injectable manufacturing technologies
• Strong cGMP-driven quality culture
• Long-term career growth and skill development opportunities
• Collaborative work environment with experienced professionals
FAQs
Which departments are hiring in this walk-in interview?
Production, Engineering, Warehouse, Microbiology, and Quality Assurance departments are hiring.
Is experience in injectables mandatory?
Yes, only candidates with experience in injectables will be considered, except for specific fresher roles mentioned.
Are freshers eligible for this walk-in?
Yes, freshers are eligible for selected Production roles such as packaging and training-related positions.
What is the work location after selection?
The work location will be Nagarjuna Sagar, Telangana.
What qualifications are accepted for Production roles?
Diploma, BSc, BPharm, and MSc are accepted depending on the role.
What experience range is required overall?
Experience requirements range from freshers up to 12 years, depending on department and role.
What are the interview timings?
The interview will be conducted from 09:00 AM to 02:00 PM.
Do candidates need to bring documents?
Yes, candidates must bring an updated resume, educational certificates, experience proofs, salary slips, and ID proof.
Are rotational shifts required?
Yes, selected candidates must be willing to work in rotational shifts.
Is this walk-in only for Hyderabad-based candidates?
Candidates from any location can attend, provided they are willing to work at the Nagarjuna Sagar site.
 Follow us on LinkedIn
Follow us on LinkedIn
