Company overview
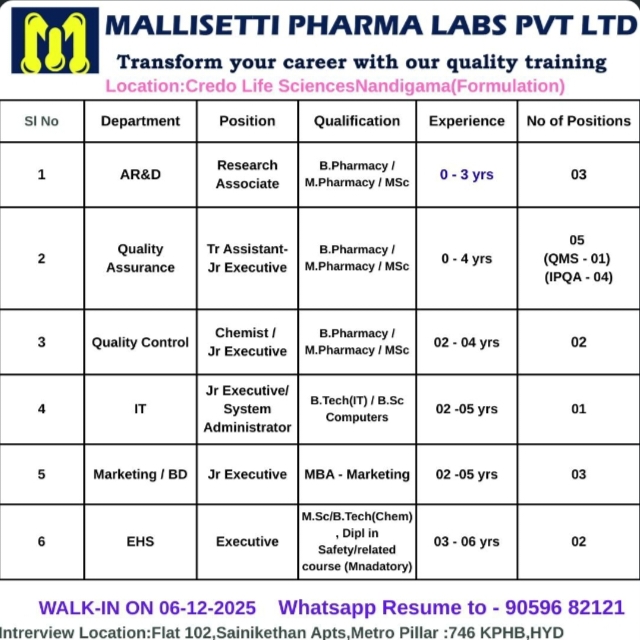
Pharma jobs at Mallisetti Pharma Labs offer a strong opportunity for candidates who want to work in AR&D, QA, QC, IT, Marketing and EHS within a growing pharmaceutical setup. The company works closely with Credo Life Sciences at Nandigama and focuses on high-quality formulation processes, compliance and industry-standard training. These pharma jobs help candidates gain hands-on experience and long-term growth in technical and non-technical functions.
Job details
Departments and Positions
AR&D – Research Associate
Quality Assurance – Trainee Assistant, Junior Executive
Quality Control – Chemist, Junior Executive
IT – Junior Executive, System Administrator
Marketing / Business Development – Junior Executive
EHS – Executive
Location
Credo Life Sciences, Nandigama (Formulation)
Requirements
Skills
Perform assigned tasks as per department SOPs
Maintain documentation as required
Support daily operations in AR&D, QA and QC
Ensure compliance with regulatory guidelines
Use computer systems for IT and documentation
Coordinate with internal teams
Follow safety practices
Adapt to dynamic work requirements
Qualifications
B Pharmacy
M Pharmacy
MSc
B Tech (IT)
BSc Computers
MBA Marketing
MSc / B Tech (Chem) with Diploma in Safety (Mandatory for EHS)
Experience
AR&D: 0–3 years
Quality Assurance: 0–4 years
Quality Control: 2–4 years
IT: 2–5 years
Marketing / BD: 2–5 years
EHS: 3–6 years
Vacancies
AR&D – 03
QA – 05
QC – 02
IT – 01
Marketing / BD – 03
EHS – 02
Interview details
Type
Walk-in Interview
Date
06 December 2025
Location
Flat 102, Sainikethan Apartments
Metro Pillar 746, KPHB, Hyderabad
Contact
WhatsApp resume to: 90596 82121
Documents required
Updated resume
Educational certificates
Experience letters
ID proof
Passport photo
Safety course certificates (for EHS)
Any additional supporting documents
How to apply
Eligible candidates can attend the walk-in interview directly with all required documents. These pharma jobs offer good exposure for candidates aiming to build a career in quality, research, IT or safety functions.
 Follow us on LinkedIn
Follow us on LinkedIn
