Introduction:
Emcure Pharma jobs for API Quality, MSc BE are opening a strong walk-in drive opportunity for experienced professionals looking to grow their careers in the active pharmaceutical ingredients segment. This hiring drive is specially organized for API Quality roles at Emcure’s well-established Kurkumbh and Pimpri facilities, offering excellent exposure to regulated environments, advanced quality systems, and long-term professional growth.
Company overview:
Emcure Pharma jobs for API Quality, MSc BE are offered by Emcure Pharmaceuticals Ltd., a reputed Indian pharmaceutical company known for its strong presence in API and finished dosage manufacturing across global markets. Emcure is recognized for its commitment to quality, compliance, innovation, and patient-centric healthcare solutions. With modern manufacturing facilities in Maharashtra and a culture focused on continuous improvement, the company provides stable career opportunities for professionals in quality control, quality assurance, microbiology, and IT quality systems.
Job details:
Job Title:
API Quality Control Executive
API Microbiology Executive
API Quality Assurance Executive
API ITQA Executive
Department:
API Quality Control, API Microbiology, API Quality Assurance, API ITQA
Salary:
N/A
Requirements:
Skills:
Handling of raw materials, intermediates and finished products
HPLC, GC, ICPMS, Empower and Chromeleon software handling
Particle size analyzer and LCMS GCMS knowledge
Microbial analysis including water analysis and MLT
Environment monitoring and media preparation
API quality assurance activities
In-process QA and API line clearance
QMS documentation handling
Deviation management, change control and CAPA
GxP IT and computer system validation activities
Software qualification and user access management
Risk assessment and regulatory compliance knowledge
Qualification:
MSc Analytical Chemistry, MSc Organic Chemistry, MSc Microbiology, BE Computers, BE Electronics and Telecommunication
Experience:
1 to 6 years
Vacancies:
Not yet disclosed
Interview Details:
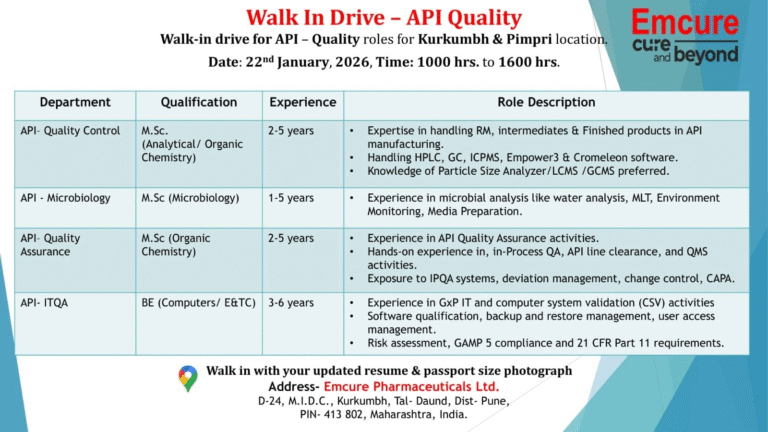
- 📁 Department: API Quality Control
📄 Qualification: MSc Analytical Chemistry, MSc Organic Chemistry
📄 Experience: 2 to 5 years - 📁 Department: API Microbiology
📄 Qualification: MSc Microbiology
📄 Experience: 1 to 5 years - 📁 Department: API Quality Assurance
📄 Qualification: MSc Organic Chemistry
📄 Experience: 2 to 5 years - 📁 Department: API ITQA
📄 Qualification: BE Computers, BE E and TC
📄 Experience: 3 to 6 years
Type:
Walk-In Interview
Date and Time:
22nd January 2026
10:00 hrs to 16:00 hrs
Location/Platform:
Emcure Pharmaceuticals Ltd.
D-24, M.I.D.C., Kurkumbh
Tal-Daund, Dist-Pune
PIN-413 802, Maharashtra, India
Documents Required:
Updated CV/Resume
Passport size photograph
Education certificates
Government ID proof
How to Apply:
Interested candidates should directly walk in with their updated resume and passport size photograph to the mentioned address on the scheduled date and time. Candidates are advised to reach the venue early and carry all required documents for a smooth interview process.
Emcure Pharma jobs for API Quality, MSc BE offer an excellent platform for professionals who want to work in a highly regulated API manufacturing environment with exposure to advanced analytical techniques, quality systems, and compliance-driven operations. The company encourages continuous learning, teamwork, and professional development, making it a preferred employer in the pharmaceutical industry.
Emcure Pharma jobs for API Quality, MSc BE are ideal for candidates who are passionate about quality excellence, regulatory compliance, and long-term career stability. Applicants should prepare well, dress professionally, and confidently present their technical expertise during the walk-in interview to make the most of this opportunity.
 Follow us on LinkedIn
Follow us on LinkedIn
