Introduction:
Amoli Organics Pharma jobs for Production QC Quality Control Maintenance BSc MSc BE BTech are creating excellent career opportunities for skilled and passionate pharma professionals looking to grow in the API manufacturing industry. This walk-in drive is a great chance for freshers and experienced candidates to join a reputed pharmaceutical organization known for quality, innovation, and global standards. Candidates seeking stable careers in manufacturing, quality, and engineering roles should not miss these Amoli Organics Pharma jobs for Production QC Quality Control Maintenance BSc MSc BE BTech openings.
Company overview:
Amoli Organics Pharma jobs for Production QC Quality Control Maintenance BSc MSc BE BTech come from a well-established pharmaceutical company with a strong industry reputation. Amoli Organics, a division of Umedica Laboratories Pvt. Ltd., is a leading manufacturer of Active Pharmaceutical Ingredients (APIs) with headquarters in Mumbai and a major manufacturing site at GIDC, Vapi. The company has been working with global healthcare leaders for over three decades and operates facilities approved by international regulatory bodies such as USFDA and EDQM. Known for advanced process chemistry, strict GMP compliance, and a strong quality culture, Amoli provides a professional environment where employees gain hands-on exposure to modern API manufacturing technologies and global quality systems.
Job details:
Amoli Organics Pharma jobs for Production QC Quality Control Maintenance BSc MSc BE BTech offer multiple career paths across core manufacturing and support functions in API production.
Job Title:
Officer / Sr. Officer
Associate / Jr. Officer
Apprentice / Jr. Associate
Executive / Sr. Executive / Mechanical Engineer / Utility Engineer
Department:
Production
Production and Quality Control
Quality Control
Maintenance and Engineering
Salary:
N/A
Requirements:
Skills:
Knowledge of API plant handling equipment like reactor, centrifuge, dryer, and utility operations
Understanding of GMP and good documentation practices
Knowledge of HPLC, GC, Autotitrator, UV, FTIR, Chromeleon 7.2, LIMS, and related QC instruments
Maintenance of API and chemical plant equipment such as RBL, SSR, CVD, CVPD, HVAC, chiller, brine, nitrogen, boiler, air compressor, and plant maintenance systems
Troubleshooting of mechanical and utility equipment in regulated manufacturing environments
Qualification:
MSc Organic Chemistry
BSc Chemistry
BE Mechanical
BTech Mechanical
Experience:
Freshers
1–3 years
2–6 years
4–8 years
Vacancies:
37 plus additional positions not yet disclosed
Interview Details:
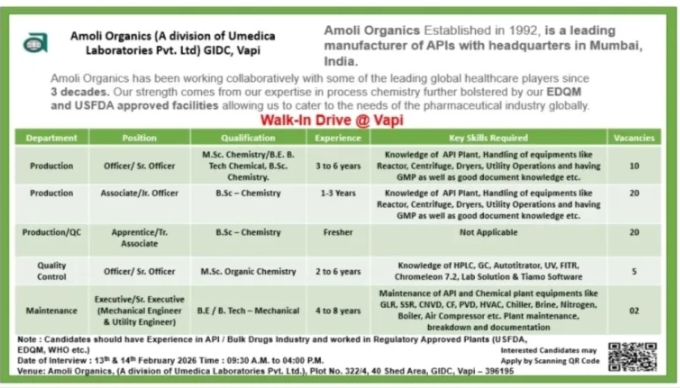
- 📁 Department: Production 📄 Qualification: MSc Organic Chemistry, BSc Chemistry 📄 Experience: 3–6 years
- 📁 Department: Production 📄 Qualification: BSc Chemistry 📄 Experience: 1–3 years
- 📁 Department: Production and Quality Control 📄 Qualification: BSc Chemistry 📄 Experience: Fresher
- 📁 Department: Quality Control 📄 Qualification: MSc Organic Chemistry 📄 Experience: 2–6 years
- 📁 Department: Maintenance and Engineering 📄 Qualification: BE Mechanical, BTech Mechanical 📄 Experience: 4–8 years
Type:
Walk-In Interview
Date and Time:
13 February 2026 – 14 February 2026
09:30 AM to 04:00 PM
Location/Platform:
Amoli Organics (A division of Umedica Laboratories Pvt. Ltd.), Plot No. 3224, 40 Shed Area, GIDC, Vapi – 396195, Gujarat
Documents Required:
Updated CV/Resume
Educational Certificates
Experience Certificates
Passport Size Photographs
Government ID Proof
How to Apply:
Candidates interested in Amoli Organics Pharma jobs for Production QC Quality Control Maintenance BSc MSc BE BTech can directly attend the walk-in interview at the given venue on the mentioned dates and time. It is advisable to arrive early, dress professionally, and carry all original documents along with photocopies for smooth verification. These opportunities provide exposure to regulated API manufacturing, advanced equipment, and a strong learning culture, making this a valuable career move for pharma professionals aiming for long-term growth in the pharmaceutical industry.
 Follow us on LinkedIn
Follow us on LinkedIn
