Introduction
pharma jobs seekers: this walk-in interview at AMI Lifesciences is a focused opportunity for professionals in production, engineering and quality control to join an API/formulation environment that values safety, documentation and cGMP practices.
Company overview
pharma jobs with AMI Lifesciences give candidates a chance to work in a modern formulation and API-support setting. AMI is known for structured production practices, robust quality systems, and strong emphasis on regulatory compliance and employee training. The company promotes hands-on skill development, cross-functional teamwork and career progression in manufacturing and technical roles.
Job details
Job Title
Trainee / Junior Executive / Executive / Senior Executive / Jr Engineer / Manager
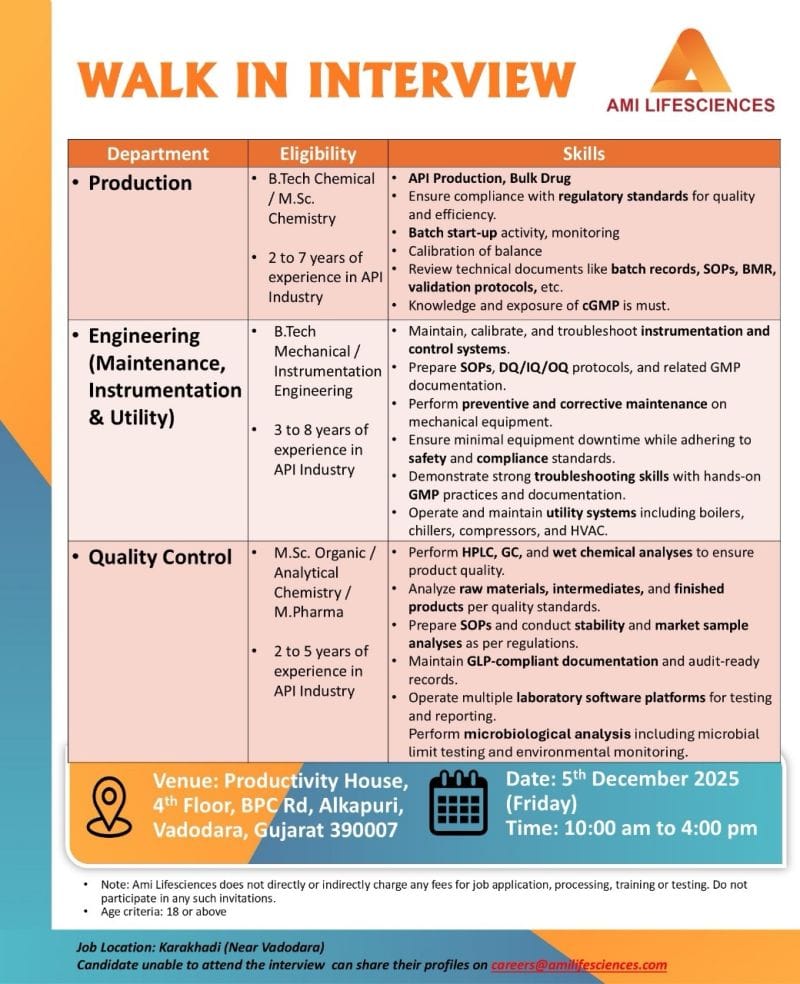
Department
Production
Engineering (Maintenance, Instrumentation & Utility)
Quality Control
Salary
N/A
Requirements
Skills
API production and bulk drug operations
Batch start-up activities and monitoring
Calibration and balance handling
Review of batch records, SOPs, BMR, validation protocols
Knowledge and exposure to cGMP
Troubleshooting instrumentation and control systems
Prepare DQ/OQ/IQ protocols and GMP documentation
Preventive and corrective maintenance on mechanical equipment
Operate utility systems (boilers, chillers, compressors, HVAC)
Perform HPLC, GC and wet chemical analyses
Microbiological testing and environmental monitoring
Qualification
B.Tech (Chemical)
M.Sc. Chemistry / M.Pharm
Experience
Production: 2 to 7 years in API industry
Engineering: 3 to 8 years in API industry
Quality Control: 2 to 5 years in API industry
Vacancies
Not yet disclosed
Interview Details
Type
Walk-In Interview
Date and Time
05 December 2025, 10:00 am to 04:00 pm
Location/Platform
Productivity House, 4th Floor, BPC Road, Alkapuri, Vadodara, Gujarat 390007
Documents Required
Updated CV/Resume
Educational certificates (original + photocopies)
Experience letters / relieving letters
ID proof (Aadhaar/Passport/Driving Licence)
Passport size photographs
Any relevant safety or validation certificates
How to Apply
Attend the walk-in interview at the venue with all documents or share your profile by email to careers@amilifesciences.com. Candidates unable to attend may send detailed resumes to the email address for consideration.
Extra tips for candidates
Arrive at least 30 minutes early and carry originals plus copies of documents. Dress professionally and prepare to discuss specific batch operations or instrumentation troubleshooting. Emphasize any hands-on experience with cGMP, validation work, and utility systems. These pharma jobs reward attention to documentation, safety, and teamwork.
Closing
If you are ready to grow in a regulated production environment, prepare your documents and apply confidently for these pharma jobs at AMI Lifesciences.
 Follow us on LinkedIn
Follow us on LinkedIn
